Abstract
Background: ATHN 7, A Natural History Cohort Study of the Safety, Effectiveness, and Practice of Treatment for People with Hemophilia (NCT03619863), monitors the use of current hemophilia A (HA) and B therapies, including the substitution therapy, emicizumab. Emicizumab is a bispecific monoclonal antibody that bridges activated factor (F)IX and FX, substituting for the function of deficient activated FVIII in people with hemophilia A (PwHA). Emicizumab is approved in the United States for prophylaxis in PwHA ages newborn and older, with or without FVIII inhibitors. The population of females with HA is rare and under-represented in the medical literature. This analysis aims to characterize the females with HA enrolled in ATHN 7 who were receiving emicizumab and present their associated experience on this therapy, including the safety profile of emicizumab in this population.
Methods: ATHN 7 is a longitudinal, prospective observational cohort study conducted at 26 American Thrombosis and Hemostasis Network (ATHN)-affiliated sites. Ethics approval was obtained and participants and/or parents/guardians provided consent. Females (assigned sex at birth) with HA receiving care at participating sites were eligible for inclusion. Demographic information was collected at baseline. Clinical information was collected at baseline and at least quarterly through participant interview and medical record review. Descriptive statistics of medical history and demographic data as well as longitudinal data were used to characterize the study population. Adverse events (AEs) were documented.
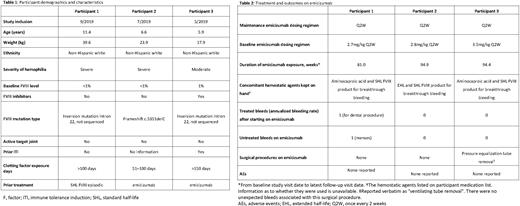
Results: As of December 31, 2021, demographic information was available for three emicizumab-treated females with HA in ATHN 7 (Table 1). One of the three was postmenarchal (11 years of age), while the other two were premenarchal. Two of the participants had baseline factor FVIII activity of less than 1% (classified as severe HA) and no history of FVIII inhibitors, while the third participant had a baseline FVIII activity of 1% (classified as moderate HA) and a history of tolerized low titer FVIII inhibitors. Two participants (one with severe HA, and one with moderate HA with FVIII inhibitors) had a FVIII inversion mutation in intron 22 and the third participant (with severe HA) a FVIII frameshift mutation c.5351delC. All three participants had >50 exposure-days to FVIII at study entry and no target joints present. There were no other reported hemophilia-related concomitant conditions in any of the three participants in this analysis.
Participants received emicizumab once every 2 weeks (Table 2). Emicizumab exposure ranged from approximately 81.0 to 94.9 weeks, the longest follow-up in female participants reported to date in the literature. The data represent a total of 5.18 patient-years exposure to emicizumab. Only one participant experienced a treated bleed, which was associated with a dental procedure. This participant also reported one bleed that was not treated (menses). There were no other treated or untreated bleeds reported in these participants. During the study, one participant underwent surgery (pressure equalization tube removal), with no unexpected bleeds associated with the procedure. No AEs were reported for any of the three female participants while being treated with emicizumab.
Conclusions: This analysis contributes to filling the data gap existing for females with HA. In the largest multi-center, prospective observational experience of emicizumab-treated PwHA in the US (ATHN 7), two of the three female participants had no bleeds (treated or untreated), while the third participant had one treated bleed associated with a dental procedure and one untreated bleed associated with menses. No AEs were reported. Continued data collection is vital to further understand safety and effectiveness in this rare and under-represented population to optimize care for females with HA.
Disclosures
Recht:American Thrombosis and Hemostasis Network; Yale University School of Medicine: Current Employment; Oregon Health & Science University: Ended employment in the past 24 months; Catalyst Biosciences, CSL Behring, Genentech, Grifols, Hema Biologics, Novo Nordisk, Pfizer, Sanofi, Takeda, uniQure: Consultancy; Bayer, Biomarin, CSL Behring, Genentech, Grifols, Hema Biologics, LFB, Novo Nordisk, Octapharma, Pfizer, Sanofi, Spark Therapeutics, Takeda, uniQure: Research Funding; Foundation for Women and Girls with Blood Disorders; Partners in Bleeding Disorders: Thrombosis and Hemostasis Societies of North America: Membership on an entity's Board of Directors or advisory committees. Daoud:American Thrombosis and Hemostatsis Network: Current Employment. Lee:Genentech, Inc: Current Employment, Current equity holder in publicly-traded company. Morton:Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company. Raffini:Genentech, Inc.: Membership on an entity's Board of Directors or advisory committees; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Childrens Hospital of Philadelphia: Current Employment; Boeringer Ingelheim: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal